A team of Harvard researchers have designed a human airway chip that can mimic the lungs of patients with cystic fibrosis.
Cystic Fibrosis (CF) results from a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. This causes production of thick and sticky mucus within a person’s airways and other body organs. Thus, resulting in repeated respiratory infections, inflammation, lung scarring and eventually respiratory failure. Current therapeutics have considerably increased the life expectancy of CF patients. However, the lack of human in-vitro models makes it difficult to completely understand the disease pathology and develop newer, targeted therapies. To solve this challenge, researchers at Harvard’s Wyss Institute have designed a lung airway chip that mimics that of CF patients.
The bundled capabilities of this advanced in vitro model can help accelerate the search for drugs that may dampen the exaggerated immune response in patients, treat them with more personalized therapies, and help solve problems that CF patients face every day which cannot be addressed by existing treatments.
– Dr. Donald Ingber
The team designed the USB-sized microfluidic device using lung airway cells from cystic fibrosis patients. One of the hollow channels contained airway cells while in the second they recreated human blood vessels and perfused it with a blood substitute medium. Moreover, both the hollow channels were separated by a porous membrane. They then compared it to a control airway chip that contained healthy lung cells.
The Future of Drug Testing
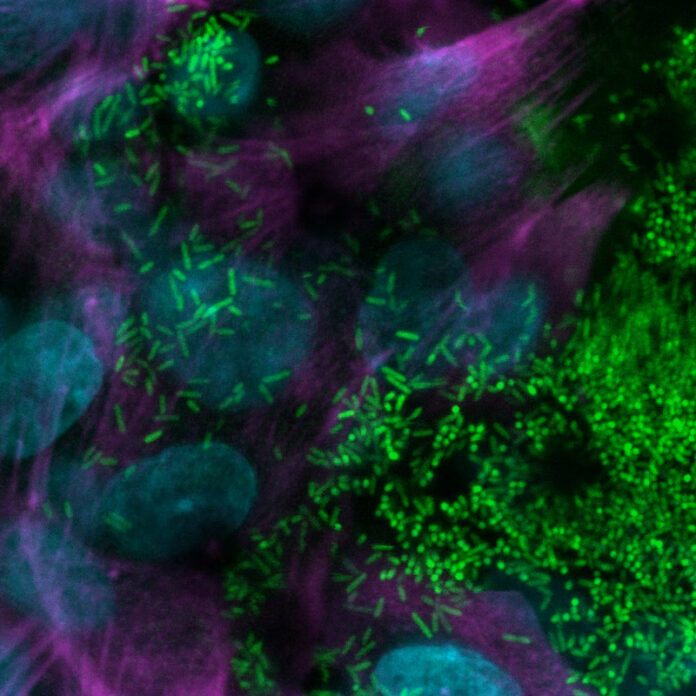
After two weeks of culture, the CF airway chip demonstrated multiple features of CF pathology. These included a thicker mucus layer, increased cilia density, and a higher ciliary beating frequency. Researchers also noted greater levels of pro- and anti-inflammatory factors within the model as compared to the control chip. These factors are generally involved in driving inflammation in lungs of CF patients.
When researchers observed the flow of these immune cells within the channels, they noted a greater accumulation in the airway epithelial cell layer. Moreover, the CF airway chip demonstrated a more favorable environment for growth of Pseudomonas aeruginosa.
According to the study published in Journal of Cystic Fibrosis, this is the first airway model to closely mimic that of a CF patient. Thus, providing researchers with an effective preclinical human model for investigating new CF therapies.
However, this is not the first time that organ-on-a-chip technology has been used to study disease pathology. Recently, researchers at Wyss Institute designed a human intestine chip to study the effects of anti-COVID drugs on the gut. The advancement in chip technology can likely provide researchers with a new avenue for drug testing.
Reference:
Plebani, Roberto, et al. “Modeling Pulmonary Cystic Fibrosis in a Human Lung Airway-on-a-Chip.” Journal of Cystic Fibrosis, 2021, doi:10.1016/j.jcf.2021.10.004.