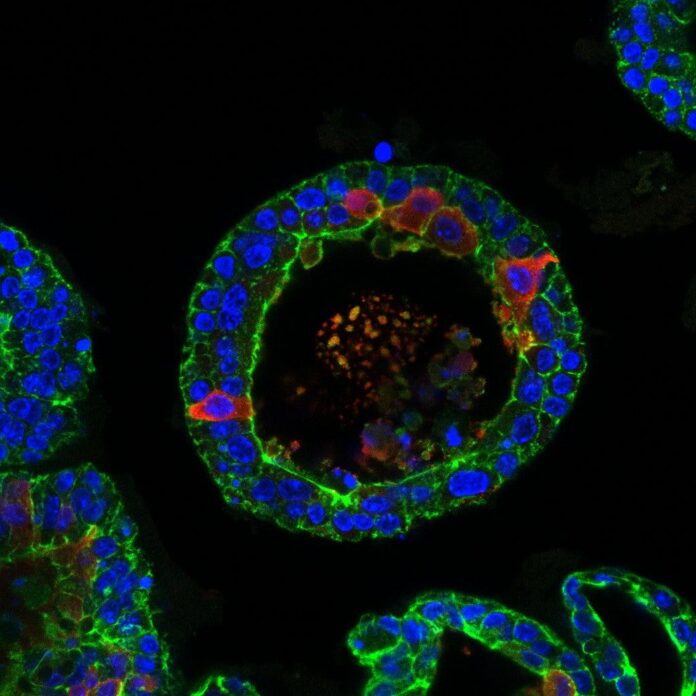
For the first time, scientists have induced artificial tear glands, created from human stem cells, to cry.
Your tear glands have probably been working on overtime during the pandemic. While these tears helped you cope with some of your emotions, they also kept your eyes lubricated and protected. However, in around 5% of the world’s population these tear glands are unable to provide them with this added benefit. Certain conditions can lead to reduced tear productions in people; thus, causing dry eyes and significant discomfort. Moreover, treatment options for such eye conditions are limited as they are poorly understood. Therefore, researchers in Netherlands created artificial tear glands derived from human stem cells. With the aim to help develop regenerative therapies.
The researchers described their research in a paper published in Cell Stem Cell.
The team first grew mouse and human lacrimal organoids using adult stem cells. Organoids are small 3D structures that resemble the functions of the full organ. They then exposed the mouse organoid to noradrenaline, a neurotransmitter that triggers the release of tears. This caused the tear glands in the dish to swell up like a balloon as the cells released tears.
The challenge was to get the organoids to cry, as this is a hallmark of the lacrimal gland. We had to modify the cocktail of factors the organoids are grown in so that they would become the mature cells that we have in our tear glands and that are capable of crying.
Marie Bannier-Hélaouët, study author

Why Do We Cry?
Tear glands, also called lacrimal glands, are responsible for secreting our flowing tears. This liquid film covers the eyes in lubricating fluid, while its antibacterial properties help it protect the eyes. Production of tears occurs in response to the secretion of neurotransmitters, acetylcholine and noradrenaline. Any disruption in production or secretion of tears results in painful, dry eyes. Moreover, certain autoimmune conditions such as Sjögren’s syndrome can also result in dry eyes. Doctors usually prescribe eye drops or surgery as a treatment. Thus, with their study, doctors hope to increase the available options.
Using single-cell mRNA sequencing, the researchers could identify different components of the artificial human tear glands. They discovered that the two type of cells in the glands, duct and acinar, produce different components of the tears.
Once the researchers successfully induced tear production in the artificial tear glands, they investigated the genes responsible for functioning of the glands. Using gene editing techniques, they deleted the gene PAX6 from the mouse organoids. Deficiency of this particular gene is considered a possible cause of Sjögren’s syndrome. This resulted in the cells losing their ability to differentiate.
We hope that scientists will use our model to identify new treatment options for patients with tear-gland disorders by either testing new drugs on a patient’s organoids or expanding healthy cells and, one day, using them for transplantation
Hans Clevers, senior author
The researchers further tested the organoids’ potential for use in regenerative medicine. They transplanted the human organoid cells into mouse lacrimal glands. This resulted in the cells integrating into the gland, and the formation of duct cells. These structures remained for at least two months. Moreover, some of the human cells continued to grow and divide up to two months after transplantation. Researchers also identified tear proteins within the ducts formed from the transplanted cells.
However, there’s still a long way to go before the method can be applied to humans.
Reference:
Bannier-Hélaouët et al., Exploring the human lacrimal gland using organoids and single-cell sequencing, Cell Stem Cell (2021), https://www.cell.com/cell-stem-cell/fulltext/S1934-5909(21)00075-8



