Dr. Harald Enzmann considered various career options including history and politics. He chose medicine for merely two reasons: he aimed to do something meaningful and most importantly, he was aware of the broad range of specialities that medicine allows a young doctor to pursue. From patient care and research to exotic ideas about the history of medicine, the possibilities are endless. With this broad range, he didn’t have to think about where he would end up. Finally, he ended up in the pharmaceutical industry!
During the COVID-19 crisis, Europe’s public looked up to Dr. Enzmann for he chaired the Committee for Medicinal Products for Human Use (CHMP) – responsible for launching the first COVID-19 vaccine in Europe.
Chair of Committee for Medicinal Products for Human Use (CHMP) of European Medicines Agency (EMA)
In 1985, Dr. Harald Enzmann pursued a Doctorate of Medicine (MD) from Karl Ruprecht’s University, Heidelberg. In 1995, he obtained a Master of Science Degree (MS) in Experimental Pathology from New York Medical College, USA. His career began in Bayer Healthcare where he served as Laboratory Head and Division Head. He worked on toxicology, genotoxicity, carcinogenicity, and rodent studies for pharmaceuticals, agrochemicals and chemicals. In 2002, Dr. Enzmann joined Federal Institute for Drugs and Medical Devices (BfArM). He served as Division Head of Preclinical Pharmacology and Toxicology and later as Head of licensing division. His untiring efforts and unmovable dedication to the institute promoted him to Section Head of European & International Affairs. Federal Institute for Drugs and Medical Devices (BfArM) runs the Committee for Medicinal Products for Human Use (CHMP).
Since 2005, Dr. Enzmann has been serving as a proud member of CHMP. After being a dedicated member of CHMP for 15 years, in 2016, he was elected as Vice-Chair of CHMP. With outstanding performance and critical involvement in the affairs of the institute, Dr Enzmann was re-elected as Chair of CHMP in 2018.
After serving the committee for several years, I wanted to chair the committee. My colleagues believed “Harald could do it”. I feel immensely proud to have the opportunity to serve as Chair of CHMP. Interestingly, there are other committees where a chair votes as well. If the elections end up in a tie it is his vote that decides. This does not happen in BfArM. Here, the chair does not vote. So, honestly, I am extremely thankful that even in the re-election I got all the votes. This is one of the most gratifying moments!
Dr. Harald Enzmann
What does the Chair of CHMP do?
The Committee for Medicinal Products for Human Use (CHMP) is one of the highest regulatory bodies of the European Medicines Agency (EMA). This committee assembles 50 experts who conduct meetings lasting for several days to provide opinions on new medicines. Review and evaluation of a huge amount of data and numerous documents and proposals for and against the certification and authorization of the medicinal product are presented to the members of the meeting. The final report is forwarded to the EU Commission for formal approval.

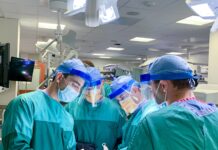
Dr. Harald Enzmann with COVID-19 Frontliners in Meeting Rooms
As the Chair of CHMP, the biggest challenge was the COVID-19 crisis. When the pandemic hit Europe, it was a state of emergency and everything was unclear. There was a dramatic increase in the demands and expectations from the committee. With the then standard of protocol, it would have been 10 years until the first vaccine or medicine for COVID-19 came out. Dr. Enzmann and his team decided to change the way everything happened and handle matters differently.
In the COVID-19 crisis, standard timetables and monthly meetings flew out of the window. Moreover, we needed to work in a legal frame that was never tested for times of crisis. We had to build new ways to interpret legal frames differently and speed matters up. With dramatic action, vaccines became available. The duration between receiving a medical product and providing an opinion on it had reduced intensely. It was before Christmas 2019 when the committee decided to pace up and do better. On the 20th of December of 2019, we launched the first COVID-19 vaccine in Europe.
Dr. Harald Enzmann
Maze of Right Choice
Dr. Harald Enzmann didn’t achieve his position at the pharmaceutical regulatory authority overnight. He had his share of professional experience. He served at the German Cancer Research Center, Institute of Pharmacology and Toxicology at the University of Erlangen, at R&D at Bayer AG, Wuppertal and the American Health Foundation in Valhalla, NY, USA, Associate Lecturer for Pharmaceutical Medicine and member of the Examination Board at the University Duisburg-Essen. This is not all! He is also a fellow of the International Academy of Toxicologic Pathology, a member of the European Society of Toxicological Pathology, Society of Toxicology and European Association for Cancer Research.
I think I am one of the limited number of people in this world who can do what they are interested in. I started from clinical practice and research and finally ended up on the Committee for Medicinal Products for Human Use (CHMP). The best quality of my work is my ability to do what I like to do. There are times when I have to do something I don’t like. But, the majority of the time I do what fascinates me.
Dr. Harald Enzmann
Healthy Private Life – A sufficient backup
Dr. Harald Enzmann, a physician by profession and Chair of CHMP by career, comes across stressful times during work. Negotiations during meetings, piles of files awaiting approval, a list of drugs awaiting legal opinion, providing licences and certifying new medicines and medical devices for human use is exhausting! Fortunately, he has a backup plan ready for times of stress.
I am lucky in my private life to have sufficient backup. Work-life balance is best to work every then and now. You need it as a retreat.
Dr. Harald Enzmann
Medicine & Possible Choices
Dr. Harald Enzmann encourages young medical graduates to explore possible career paths in medicine. Medical students and graduates should be able to find what they are passionate about. If they are not interested in one field they can always switch to another without any delay.
It is challenging, but it is lots of fun! The most intriguing fact about medicine is the broad range where you can have so many possibilities to do anything. Recognize all options you have in medicine and pick what you like to do. Most of us would be best when they do what they like to do!
Dr. Harald Enzmann
Researcher, clinician, and member of a pharmaceutical regulatory body – Dr. Harald Enzmann didn’t hesitate to find the right career choice. Today, he serves as the competent Chair of the Committee for Medicinal Products for Human Use (CHMP). A successful and impactful leader, like Dr. Harald Enzmann, makes the right decisions at the right time, combats the critics and plans ahead in times of crisis. This is what makes a leader exemplary.