- Vaccines initiate an immune response in the body and prevent the development of a disease.
- The first COVID-19 vaccine approved for emergency use was Pfizer/BioNTech vaccine.
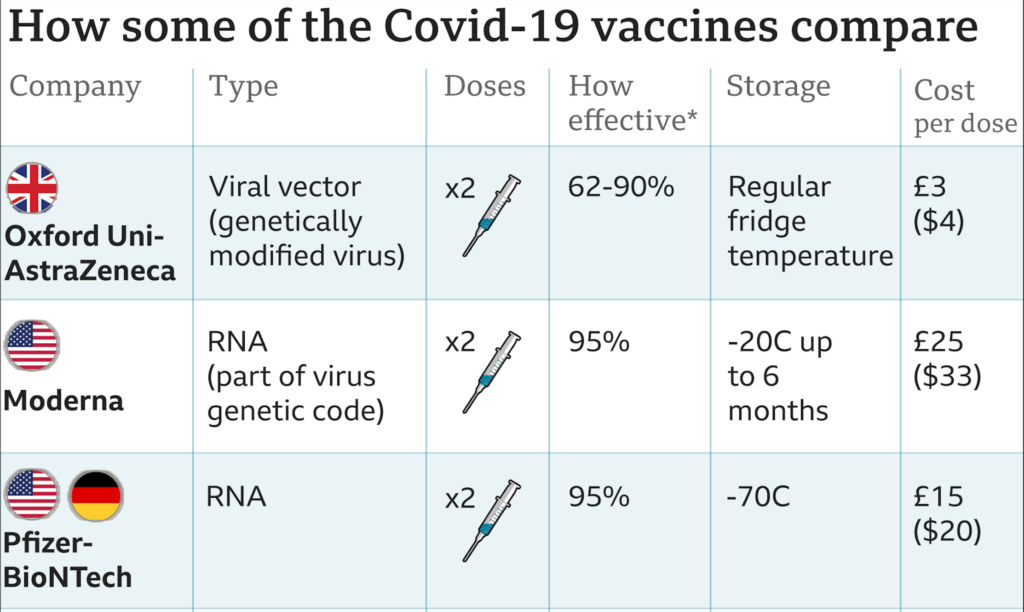
- Currently three main vaccines – Pfizer/BioNTech, Moderna, Oxford/AstraZeneca – are at the forefront of the fight against the novel coronavirus.
Since the beginning of the pandemic, scientists have been racing against time to develop potential COVID-19 vaccines. Over 60 vaccines are currently undergoing phase 3 trials across the world. Although vaccine development can take over 10 years, a few of them have already received authorization from national regulatory authorities. Furthermore, Pfizer/BioNTech vaccine has become the first vaccine listed under Emergency Use Listing by the World Health Organization (WHO).
Despite a handful of vaccines getting authorizations, three are currently in the foreground – Pfizer/BioNTech, Oxford/AstraZeneca, and Moderna. Although all three provide protection from the novel coronavirus, they all differ in their effectiveness, composition, price, and storage.
How do the COVID-19 Vaccines Work?
Both Pfizer and Moderna employ a messenger RNA (mRNA) technology. The vaccine introduces mRNA, containing instructions for the viral spike protein, inside a person’s body. The human cells then begin to create the spike protein, or the antigen. The immune system upon recognizing the antigen produced antibodies, thus, initiating an immune response. This is a particularly new technology and has never before been licensed for human use.
Unlike its competitors, the Oxford vaccine uses a viral vector to introduce the gene for the spike protein inside the human cells. In this case, the vector used is an adenovirus that causes the common cold in chimpanzees. Similar to mRNA vaccines, vector vaccines instruct the body to produce the viral spike protein, and thus trigger an immune response.
Despite a difference in technology, all three vaccines have shown a high efficacy in their respective phase 3 trials. Both Pfizer and Moderna demonstrated over 90% vaccine efficacy using a standard two-dose regimen. Whereas the Oxford vaccine showed a 70% effectiveness at preventing COVID-19. However, a regimen of half and full dose given months apart produced an efficacy of 90% among the trial participants.
How are the Vaccines Stored?
Despite Pfizer and Moderna having a higher vaccine efficacy, their storage and distribution remain a challenge. Especially in low socioeconomic regions.
Pfizer vaccine needs to be kept at -70 degrees Celsius for storage and shipment. Thus, requiring the need for ultra-cold freezers not commonly available outside of healthcare facilities. Furthermore, the vaccine can last up to five days in the refrigerator. Comparatively, Moderna can last in normal fridges for up to 30 days and at room temperature for 12 hours. Additionally, it remains stable at normal freezer temperatures for up to six months.
However, the Oxford vaccine seems the fare the best compared to Moderna and Pfizer. It can last for up to 6 months in the common household fridges. It can be sent to vaccination centers in special vaccine fridges or cool boxes. Therefore, being the easiest of vaccines to store and distribute.
The Verdict
Another factor in favor of the Oxford/AstraZeneca vaccine is its price tag. Compared to both Pfizer and Moderna it is the cheapest option costing a little under £3 per dose. Furthermore, AstraZeneca has committed to providing the vaccine at cost, without any profit, so it is available for all middle- and low-income countries.
Moreover, none of the phase 3 trials reported any severe adverse events linked to the vaccines. The most common side effects of the vaccines include pain at the injection site, fatigue, fever, and headache. Multiple cases of allergic reactions have also come forward since countries began administering the vaccines. However, none have been fatal.
Although Oxford seems to fare well compared to others, it is far too early to predict the success of the COVID-19 vaccines. As researchers continue to determine the vaccine’s lasting immunity, it is hoped that more vaccine approvals will likely result in an end to the pandemic.




