What began as a single cell later turned into a complex being composed of skin and bones, by undergoing multiple cell divisions and later differentiation. Differentiation is the process by which cells take on a certain function in a part of the body.
Stem cells are thought to be undifferentiated cells with the potential to give rise to specialized cells, including blood cells, or nerve cells, via differentiation. Their regenerative abilities make them a perfect target for regenerative medicine. Stem cell therapy, also known as regenerative medicine, promotes the repair of injured or dysfunctional tissue using stem cells or their derivatives.
An experimental study published in the journal, Nature Communications, established the use of stem cell therapy to replace and repair damaged blood vessels in the retina, due to diabetic retinopathy.
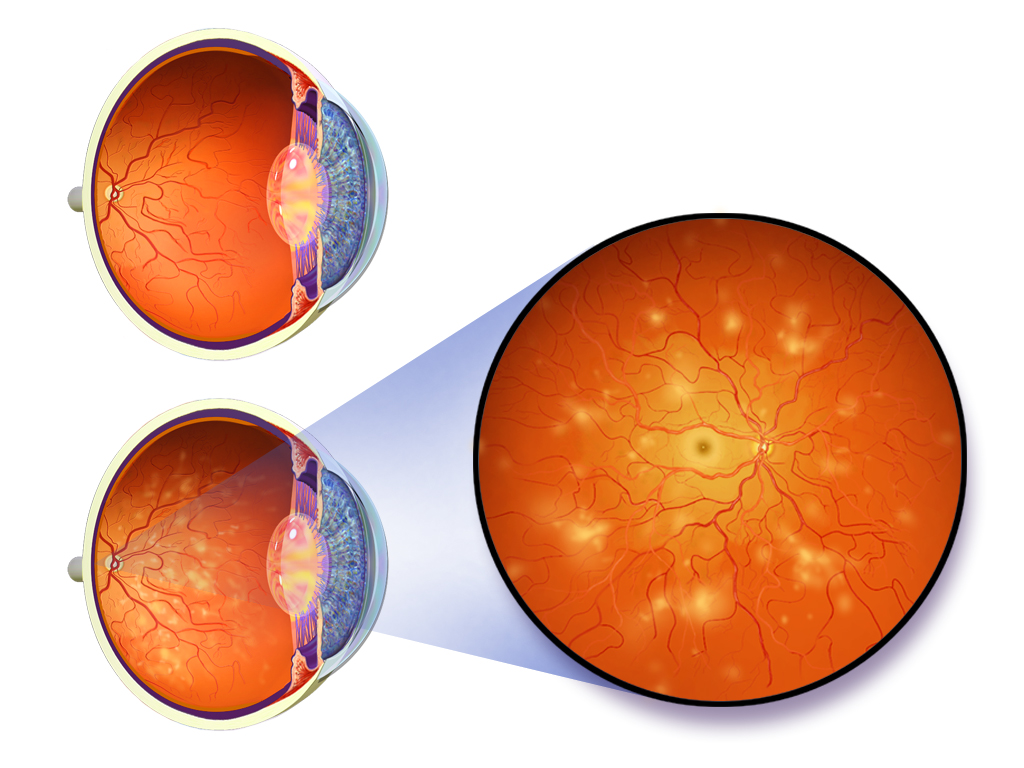
What is Diabetic Retinopathy?
Worldwide, one-third of the estimated 285 million people with diabetes show signs of diabetic retinopathy. Diabetic Retinopathy tends to develop in individuals with long-standing diabetes as a result of damage to the small blood vessels and neurons of the retina. Narrowing of the retinal arteries results in reduced retinal blood flow. The lack of oxygen in the retina causes fragile, leaky blood vessels to grow along the retina and in the inside of the eye which can result in blurry vision and ultimately blindness.
The team of researchers at John Hopkins University School of Medicine reprogrammed fibroblasts, taken from a type 1 diabetic, to function as stem cells. Using a cocktail mixture composed of MEK, GSK3β and PARP inhibitors, called 3i, they were able to reprogram the fibroblast stem cells into a primitive state more similar to embryonic cells 6 days after fertilization, than the usual human-induced pluripotent stem cells (hiPSC). These ‘naive’ cells are said to be more capable of developing into any specialized type of cell than a conventional hiPSC.
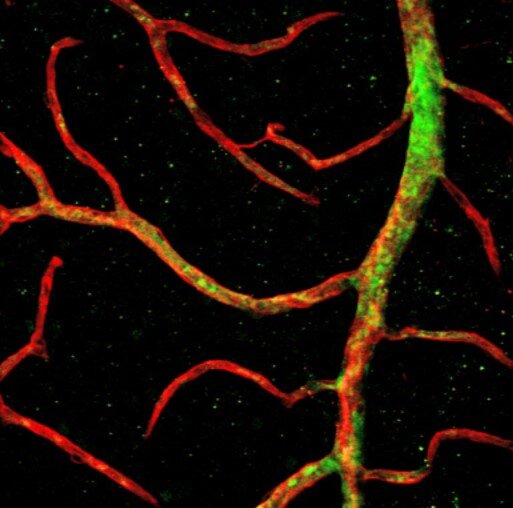
Vascular progenitors capable of forming new blood vessels, made from the naive stem cells, were then injected into the eyes of mice with a form of diabetic retinopathy. These vascular progenitors were seen to migrate to the retina’s innermost layer.
According to Elias Zambidis, one of the researchers on the team, “the 3i ‘naive reprogramming’ cocktail appeared to erase disease-associated epigenetics in the donor cells, and brought them back to a healthy, pristine non-diabetic stem cell state.”
Vascular progenitor cells developed from non-naïve stem cells were seen to migrate not as deeply into the retina, nor survive the length of the study as compared to naïve stem cell-derived progenitors.
The researchers believe that the cocktail mixture, used to develop primitive stem cells, should be refined further to further advance stem cell therapy.
References:
Park, T.S., Zimmerlin, L., Evans-Moses, R. et al. Vascular progenitors generated from tankyrase inhibitor-regulated naïve diabetic human iPSC potentiate efficient revascularization of ischemic retina. Nat Commun 11, 1195 (2020)



