Exposure to contaminated bone allograft material, FiberCell exposes more than 100 spinal surgery patients to Tuberculosis.
Aziyo Biologics Inc. is a regenerative medicine company that creates products for patients undergoing various surgical procedures. Recently, the company recalled 154 containers of its bone repair product, FiberCell. The voluntary recall took place due to several patients, fitted with the product, developing post-surgical infections. Moreover, four of them tested positive for Tuberculosis (TB). According to the US Center for Disease Control and Prevention (CDC), more than 100 patients received exposure to the contaminated product. Both CDC and the FDA are currently investigating the TB outbreak.
Patient health and safety are our highest priority. Accordingly, we have implemented this voluntary recall and instructed hospitals that received FiberCel product from this specific donor lot to immediately quarantine and return any remaining product to us
Ron Lloyd, President and Chief Executive Officer at Aziyo Biologics
Human bone tissue helps make FiberCell, a bone repair product. This viable bone matrix, containing human cells, assists in various orthopaedic and spinal procedures. All 154 units of FiberCell in the recalled lot had come from a single donor. Around 37 facilities across 20 states in the United States received these products. Of the 154 units, CDC stated that 136 products were implanted in 113 patients. The patients had received FiberCell after their spinal surgery.
Graft Products Not Tested for TB
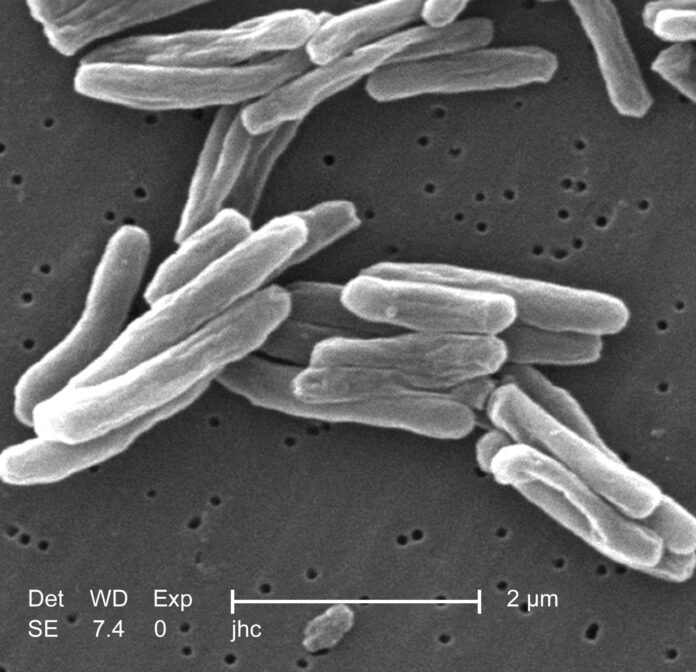
Tuberculosis is a highly contagious infection that largely affects the lungs, but can also occur in other parts of the body. Caused by the bacteria Mycobacterium Tuberculosis, the disease can remain latent in the body for years. According to the CDC, around 13 million people in the US have latent TB infection. However, the US has one of the lowest rates of TB cases in the world.
Active TB infections present with chronic cough, fever, unexplained weight loss, and night sweats. When people with active pulmonary TB talk, sneeze, cough, or spit, they release the infected aerosol droplets into the air. Thus, putting those in close ontact at risk. However, transmission through bone grafts is extremely rare. Therefore, regenerative medicine companies such as Aziyo do not test their products for the causative organism of TB.
At this point, the available evidence suggests that TB was transmitted through the product
CDC
It is unclear as to how the product may have become infected. It is hypothesized that the donor’s bone may have become infected with the bacteria during his life. And he never received treatment. Another possibility is that the donor suffered from a latent TB infection that activated later in life due to lowered immunity.
The CDC is currently recommending the standard TB treatment for all those exposed to the disease, despite no symptoms of an infection.
Source: CDC