- Although mild traumatic brain injuries often resolve with rest, at times they can lead to long-term effects such as impaired functioning.
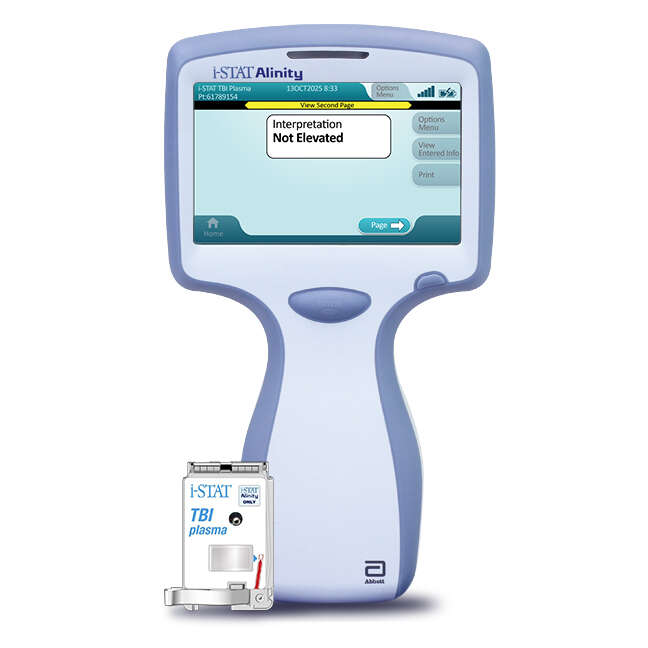
- FDA has approved the first-ever handheld blood-testing device for evaluating mild TBI.
- The device measures concussion biomarkers and can give results within 15 minutes.
The U.S Food and Drugs Administration (FDA) has approved the first-ever handheld blood-testing device for detecting traumatic brain injury. Designed by Abbot, the i-STAT™ Alinity™ TBI plasma test can potentially speed up diagnosis and improve patient outcomes.
Traumatic brain injury (TBI) is defined as damage to the brain from an external force to the head. Its most commonly caused by road traffic accidents, contact sports, and falls. Mild TBIs, also called concussions, can sometimes result in mental health disorders, memory loss, and impaired functioning. The current gold standard for diagnosing TBIs are CT scans; however, they can often miss a diagnosis. Furthermore, CT scans require transfer to a hospital which can be a time-consuming process.
Therefore, with the new device doctors hope to provide an immediate evaluation test and avoid misdiagnosis.
You can’t treat what you don’t know and now physicians will be equipped with critical, objective information that will help them provide the best care possible, allowing patients to take steps to recover, prevent re-injury and get back to doing the things they care about most.
Dr. Beth McQuiston, M.D., medical director for Abbott’s diagnostics business
Device Tests for Traumatic Brain Injury Biomarkers
Abbot’s handheld blood analyzer tests blood samples for biomarkers, UCH-L1 and GFAP. These are proteins that are often elevated in people with head trauma. Furthermore, research has found that the presence of these proteins is often a more reliable indicator of brain injury than a CT scan.
Once a blood sample from the arm is loaded onto the device’s cartridge, results are available within 15 minutes. A positive result can be followed up by a CT scan to investigate the severity of the injury and give the correct treatment. Whereas a negative result can help rule out the need for a CT scan. According to a statement released by Abbot, the device provides results with 95.8% sensitivity and greater than 99% negative predictive value. Further proving its accuracy at detecting traumatic brain injuries.
Evaluating brain injuries is complex – and research shows that we only catch about half of those who show up to the hospital with a suspected TBI. And beyond those who go to the hospital for a suspected TBI, many more never do. A test like this could encourage more people to get tested after a head trauma, which is important because not receiving a diagnosis can be dangerous and may prevent people from taking the necessary steps to recover safely.
Dr. Geoffrey Manley, M.D., Ph.D., vice chair of neurological surgery at the University of California, San Francisco
Researchers believe that the device will help provide an immediate diagnosis in point-of-care settings. Furthermore, by identifying who has an injury, doctors will be able to timely treat these patients; thus, improving patient outcomes.
Reference:
Yue, John K, et al. “Association between Plasma GFAP Concentrations and MRI Abnormalities in Patients with CT-Negative Traumatic Brain Injury in the TRACK-TBI Cohort: a Prospective Multicentre Study.” The Lancet Neurology, vol. 18, no. 10, 2019, pp. 953–961., doi:10.1016/s1474-4422(19)30282-0.
“Abbott Receives FDA 510(k) Clearance for the First Rapid Handheld Blood Test for Concussions.” Abbott MediaRoom, 11 Jan. 2021, abbott.mediaroom.com/2021-01-11-Abbott-Receives-FDA-510-k-Clearance-for-the-First-Rapid-Handheld-Blood-Test-for-Concussions.