Autologous mid-face fat grafting in Treacher Collins syndrome
Treacher Collins syndrome is defined as a condition that impairs the development of bone and soft tissue. It is an autosomal dominant disorder that affects 1 in 50,000 live births. Patients commonly present with symptoms including micrognathia and underdevelopment of the zygomatic arch. However, in the absence of any developmental delay. Micrognathia and obstruction of the hypopharynx may lead to severe difficulty in breathing. Patients diagnosed with Treacher Collins syndrome often require surgical correction of facial abnormalities and underdeveloped facial structures.
This article describes the case of a 15-year-old patient diagnosed with Treacher Collins syndrome. The patient presented to our Comprehensive Pre-anesthesia Assessment Centre for evaluation before undergoing the procedure of a bilateral autologous midface fat grafting.
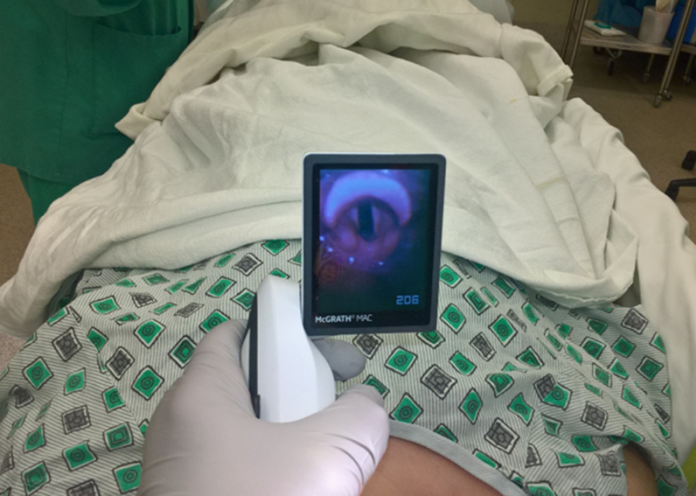
Similarly, before undergoing the procedure, the patient was also assessed to either be treated as a pediatric case or a regular surgical case. After the assessment, the patient was decided to be treated as a regular surgical case. He was also referred to an anesthesiologist for review, who further coordinated to gather photos of the patient from the primary team. In addition, he was further reviewed to gauge the severity of his airway abnormalities and possible history of difficult airway. The patient’s airway was Cormack-Lehane grade 1, a full view of the entire glottic aperture.
Anaesthetic management
Photographs from the ENT plastic surgeon showed the presence of mild ophthalmologic/otologic phenotype. Literature has shown that more than 40% of patients with Treacher Collins syndrome require direct laryngoscopy for securing an endotracheal tube. An SGA device is found to be an effective tool. After discussing with the ENT surgeon, several anesthesiologists and medical director of the outpatient surgery centre, the patient was cleared for surgery.
After a thorough review of literature, an SGA device was placed and an appropriate seal was achieved. There were no further complications and the patient’s course of treatment was also uncomplicated. The SGA device was safely removed and there were no adverse events after surgery.
Source: American Journal of Case Reports