Necessity is the mother of
invention!
With the pandemic hovering over the entire globe, each second is crucial. لعبه بينجو Therefore, since the outbreak, scientists are putting their sweat and blood in developing a breakthrough treatment.
Finally, Houston Methodist’s scientists have put to trial the nation’s first experimental plasma treatment against COVID-19.
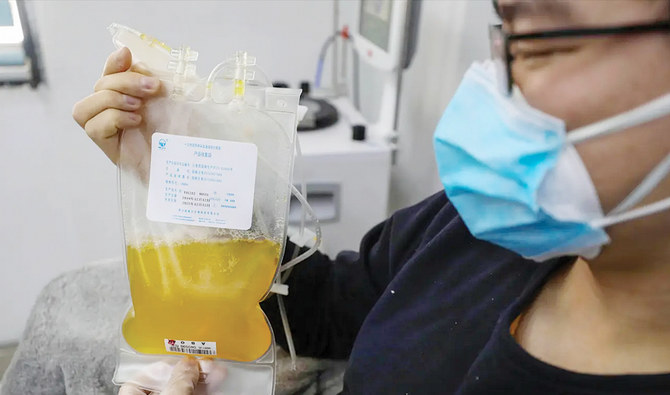
At Houston Methodist hospital, around 250 patients were recruited, who were previously positive for COVID-19 virus. Plasma was collected from the willing donors with consent and transfused to morbidly ill patients of COVID-19. This was done in the hope of recovering the antibodies made against the COVID-19 virus by the recovered patient’s immune system and transfer this plasma rich with specific antibodies into the patients whose immune system is still fighting against the novel COVID-19.
These antibodies
may be a life-saving strategy until specific vaccines and drugs surface.
Although the idea of transferring immunity between individuals hasn’t flourished theoretically, this approach, called the convalescent serum therapy, isn’t as new as it sounds. A similar concept was utilised during the pandemic Spanish flu in 1918, also the diphtheria epidemic in the 1920s, and during other infectious outbreaks. لعبة الحظ الحقيقية Even after a century, the same concept is being used. The Journal of the American Medical Association published a report describing the beneficial effects of this treatment in five COVID-19 patients in China.
“There is so much to be learned about this disease while it’s occurring; if an infusion of convalescent serum can help save the life of a critically ill patient, then applying the full resources of our blood bank, our expert faculty, and our academic medical centre is incredibly worthwhile and important to do,” said Marc Boom, the president and CEO of Houston Methodist
The
FDA has classified this convalescent serum therapy as an emergency
investigational new drug protocol (eIND). Approval from the FDA will be
required for every patient to be infused with the donated antibody-rich plasma.

What is the Process of Donating Plasma?
Have you ever donated blood? Or have you seen the process?
If yes then plasma donation is similar to blood donation. The basic difference is that for collecting plasma, the red blood cells are simultaneously returned to the donor’s body. Moreover, between blood donations there has to be a recovery period during which the red blood cells are given time to replenish, this is not the case with plasma donation. Plasma can be donated as frequently as twice per week. العاب كسب المال
After a period of 14 asymptomatic days, patients between the ages of 18 and 60 years, who had been previously diagnosed with COVID-19 are considered eligible for plasma donation.
The entire process of plasma donation, usually, doesn’t take more than 60 to 80 minutes.

The principal investigator and a physician-scientist in the Department of Pathology and Genomic Medicine at the Houston Methodist Hospital and Research Institute, Eric Salazar, M.D., Ph.D., said:
‘A review of COVID-19 patients’ charts indicates that nearly two-thirds of the patients may meet the criteria to donate plasma. Patients with critical underlying conditions and advanced age will not be eligible to donate.’
He further added:
“Convalescent serum therapy could be a vital treatment route, because unfortunately there is relatively little to offer many patients except supportive care, and the ongoing clinical trials are going to take a while. We don’t have that much time.”
This treatment has roused hopes in many hearts and has shown
some light at the end of the dark tunnel. With the world lagging months behind
a definitive cure, this plasma therapy can be the saviour.
References
Muck, P. (2020, March 28). FDA APPROVES FIRST PLASMA THERAPY FOR HOUSTON METHODIST COVID-19 PATIENT. Retrieved from Houston Methodist: https://www.houstonmethodist.org/newsroom/fda-approves-first-plasma-therapy-for-houston-methodist-covid-19-patient/