Unless you’ve been living under a rock, you must be aware of the coronavirus disease 2019 (COVID-19) that’s caused millions of people around the world to go into panic mode. On 11th March 2020, COVID-19 was declared a pandemic by the World Health Organization (WHO). Several countries since then have closed off their borders, cities have gone under lockdown, schools and offices have been closed down and people have ransacked grocery stores in preparation for what’s being considered the end of the world.
The race is now on to find a cure or a possible vaccine for a virus that has so far infected 219,573 people and caused 8,972 deaths worldwide.
We’re well prepared and focused on helping to address this evolving health situation.
Dr. Lisa Jackson, MD, MPH, senior investigator at KPWHR
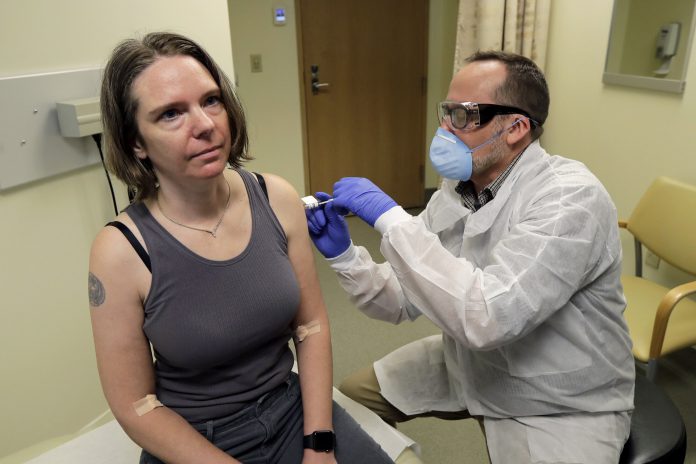
The first vaccine trial for the virus is underway at Kaiser Permanente Washington Health Research Institute (KPWHRI). The vaccine, mRNA-1273, was developed by the National Institute of Health (NIH) and a biotechnology company, Moderna Inc, and includes a short segment of messenger RNA (mRNA) that are made in the lab. There’s no chance of infection with this particular vaccine as it does not contain the coronavirus itself.
The mRNA contains the virus’s genetic code that instructs cells to create spike proteins. These spike proteins cover the surface of the virus and attach to human cells, helping it invade them. The researchers hope that once the vaccine is injected into the body, the body will start producing these harmless spike proteins, which will cause the immune system to recognize these foreign proteins and produce antibodies. Thus, in case of actual infection with the coronavirus, there will be an increased immune response to fight the invading virus.
The trial is currently at phase 1 of a 3-phase process. 45 healthy participants will be given two doses of the vaccine one month apart. The participants have been divided into 3 groups and each group will receive a different dose of the vaccine. In the first phase, researchers will test the safety of the various doses and whether they evoke an immune response. Phase 1 trials, unfortunately, will not help determine whether the vaccine is effective in preventing coronavirus infection.
Once the safety data is available in a few weeks, and the vaccine appears safe, Moderna will start phase 2 after taking permission from the FDA (Food and Drug Administration). The second phase will measure the efficacy and verify the safety of the vaccine, testing more participants than phase 1.
According to Dr. Anthony Fauci of NIH, even if the research goes well, a vaccine will still take 12 to 18 months before it becomes available for widespread use.
However, this is not the only potential vaccine in the pipeline. Many researchers around the world are working around the clock to create a vaccine against COVID-19. Another vaccine, made by Inovio Pharmaceuticals, is expected to begin its own trials next month.
Reference: https://about.kaiserpermanente.org/our-story/health-research/news/first-covid-19-vaccine-trial-at-kaiser-permanente-washington



