Anterior Uveitis 72 hours after Pfizer Vaccine: Two Cases
The FDA approved the emergency use of the Pfizer-BioNTech vaccine to prevent COVID-19. The administrative doses are two and the second dose administration is after twenty-one days.
The vaccine is recommended in people above the age of sixteen years with a 0.3ml dose. The second dose is given after twenty-one days. The immunodeficiency period is 119 days after the first jab, and it prevents severe acute respiratory syndrome because of SARS-CoV2 infection.
Currently, millions of people are getting the PfizerBioNTech vaccine across the world. However, some cases of developmental uveitis, and ocular complications following the administration of the COVID vaccine are a cause of concern. T cells play an essential role in the development of humoral vaccine response. Moreover, there is a link between the depletion with the latent herpes virus. In addition, it also represents a connection between the activation of this inflammatory response, resulting in uveitis and the vaccine.
This article presents two cases of herpetic anterior keratouveitis, seventy-two hours after the administration of the Pfizer vaccine in elderly women.
Case 1
A ninety-two-year-old Caucasian woman presented with redness in her left eye and ocular pain for two days. Three days before her symptoms she got her first jab of the Pfizer vaccine. She denied any systemic adverse reactions after the vaccine.
Her BVCA was counting two fingers at 2m and 6/10 in the right eye. In the left eye, she suffered from exudative age-related macular degeneration in LE. Her doctors treated her with intravitreal bevacizumab for four months.
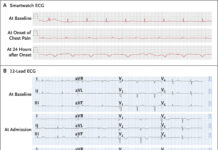
Doctors did a slit lamp examination, which revealed mild conjunctival hyperemia and intense oedema of the cornea. In addition to pigmented keratic precipitate, inferiorly and centrally, dendritic keratitis flare, and a mature Brunescent cataract.
There was no sign of hypopyon and synechiae. The intraocular pressure was 17 mmHg in the left eye and 14mmHg in the right eye. The doctors prescribed antiviral topical acyclovir five-time every day, valaciclovir 500mg every day for twenty-four hours, cyclopedic every eight hours, and moxifloxacin eye drops every four hours.
There was a remarkable improvement after four weeks of treatment.
Case 2
An eighty-five-year-old Caucasian woman came to the ER with an intense hemicranial headache on the left side in association with ocular pain in the LE.
Doctors also saw several erythematous lesions in the area supplied by the left superior ophthalmic branch of the V cranial nerve. The BCVA in the left eye was 3/10 and in the right eye 7/10.
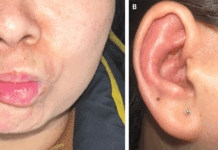
Doctors did a biomicroscopic evaluation of the left eye, which showed mild conjunctival hyperemia present in the cornea. In addition to scattered epithelial lesions with vesicular borders of small size, fluorescent positivity and flare of +/- were also seen.
All her symptoms were consistent with herpes zoster infection. Treatment included an acyclovir ointment five times daily. Doctors also prescribed oral brivudine for seven days every twenty-four hours with moxifloxacin drops every three hours.
Like Case 1, the patient in case 2 got the COVID-19 vaccine in the last seventy-two hours. However, doctors did not see any other symptoms in relation to the vaccine or any other kind either.
Discussion: COVID Vaccine-Induced Adverse Effects
The COVID-19 vaccines can cause mild adverse effects, either after the first or second jab. It includes redness, pain, swelling of the site of injection, headache, and muscle pain. People also experience nausea, vomiting, itching, chills, and joint pain. Moreover, anaphylactic shock is also a possibility, but it is very rare. The adverse effects are less with the Pfizer-BioNTech vaccine in comparison with the Moderna vaccine.
Vaccines are known to interact with the immune system, based on their design and they also activate it. However, the pathogenesis of uveitis in association with COVID-19 vaccines remains unclear.
Conclusion
This article describes the two cases of herpetic anterior uveitis after the first dose of the COVID-19 vaccine Pfizer-BioNTech.
Anterior Uveitis
The most common form of intraocular inflammation is anterior uveitis. It accounts for more than 90% of cases of uveitis. The majority of cases are seen by community ophthalmologists and 30% to 60% of them are seen in tertiary care centres.
Furthermore, most cases of anterior uveitis are idiopathic. However, they can also have an association with HLA-B27 positivity.
The causative agent of herpetic anterior uveitis is herpes simplex virus or varicella-zoster virus infection. It accounts for 5% to 10% of the uveitis cases seen in tertiary care centres. Moreover, despite the rarity, it is the most common cause of infectious anterior uveitis.
Eye Inflammation from Herpetic Reactivation
Clinicians should know that there is a possibility of eye inflammation. It is usually in the form of herpetic reactivation after the COVID-19 vaccine. In this case, it is after the Pfizer-BioNTech Vaccine in two elderly women, eighty-five and ninety-one, within 72 hours of getting the Pfizer vaccine.