A team at the University of Maryland Medicine has conducted the first-of-its-kind heart transplant using a genetically-modified pig heart.
According to organ donation statistics, 106,658 people including children and adults are currently part of the national transplant waiting list. Approximately 17 people die each day while waiting for an organ. As a result of this donor organ shortage, doctors have turned to xenotransplantation, or animal-to-human transplants. Pigs are often the preferred source as their organs are similar to humans. However, in the past doctors have only managed to conduct transplants with pig organs into brain-dead or deceased human patients. Now, for the first time, a team of doctors have managed to successfully transplant a genetically-modified pig heart into a 57-year-old man.
The successful procedure provided valuable information to help the medical community improve this potentially life-saving method in future patients.
Dr. Muhammad M. Mohiuddin, University of Maryland School of Medicine
Although pigs are biologically similar to humans, doctors don’t just use their organs as it is. This would have made xenotransplantation an easy feat. Instead, they use genetically modified pigs to prevent rejection. In this particular case, scientists made ten genetic changes involving the removal of four pig genes that produce sugar molecules and aid in rejection. Moreover, they also added six human genes. All these changes prevented excessive growth of the pig heart tissue and further aided in acceptance of the organ.
Too Early to Label Transplant ‘Successful’
David Bennett suffered from life-threatening arrhythmia and was surviving with the help of a heart-lung bypass machine. His terminal heart disease not only made him ineligible for a heart transplant but also an artificial heart pump. Therefore, a pig heart transplant remained his only available option. On December 31st, the U.S. Food and Drug Administration (FDA) granted an emergency use authorization to the historic surgery through its expanded access (compassionate use) provision.
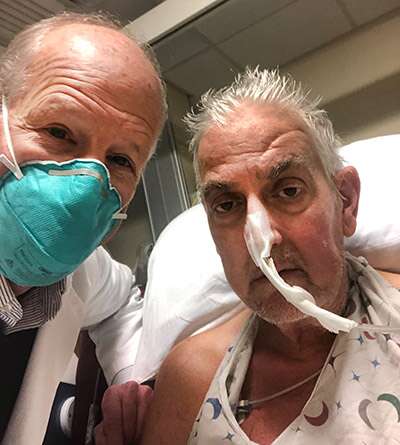
After a long eight hours, the team successfully completed the transplant. The patient is currently recovering well doctors plan to continue monitoring him for the coming weeks. Although they have not yet recorded any signs of transplant rejection, Bennett’s heart and overall health will need to be monitored for months to years, before doctors can call the heart transplant a success.
It’s working and it looks normal. We are thrilled, but we don’t know what tomorrow will bring us. This has never been done before.
Dr. Bartley P. Griffith, University of Maryland School of Medicine
Along with anti-rejection drugs, Bennett is also receiving a new experimental drug from Kiniksa Pharmaceuticals.
Source: University of Maryland School of Medicine




