Researchers at Clarkson University have developed a promising new technique to break down stubborn “forever chemicals” known as PFAS that accumulate on used water treatment media, potentially offering a simpler and cleaner way to handle contaminated materials. PFAS—per- and polyfluoroalkyl substances—are widely used in products like nonstick cookware, firefighting foams and water-resistant fabrics, but their strong carbon-fluorine bonds make them extremely persistent in the environment.
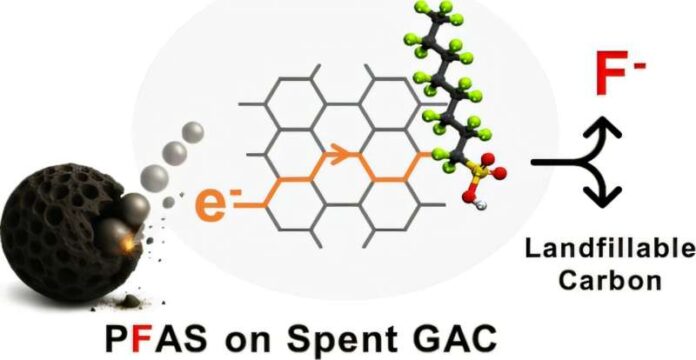
In a study published in Environmental Science & Technology Letters, the Clarkson team showed that PFAS adsorbed on granular activated carbon can be effectively destroyed using nothing more than a stainless steel ball mill. In this process, the material is placed in the mill with steel balls and mechanically ground at room temperature, without the need for added heat, solvents or chemical reagents.
The key to this method lies in the collisions between the steel balls and the carbon, which generate “triboelectrons.” These electrons interact with the PFAS molecules, helping to break their tough chemical bonds and degrade the contaminants. The approach was tested on both laboratory-prepared samples and real-world spent carbon loaded with various PFAS types, and in all cases the chemicals were destroyed.
Importantly, when the treated carbon was subjected to conditions simulating a landfill, no detectable PFAS was released, suggesting that the resulting material may be safe for disposal—an enduring challenge in PFAS cleanup efforts.
This method represents an innovative, low-energy strategy for rendering PFAS contamination less hazardous, especially for waste streams where traditional thermal or chemical destruction methods are impractical. Future work will likely focus on scaling up the technique and evaluating economic feasibility for broader environmental remediation applications.