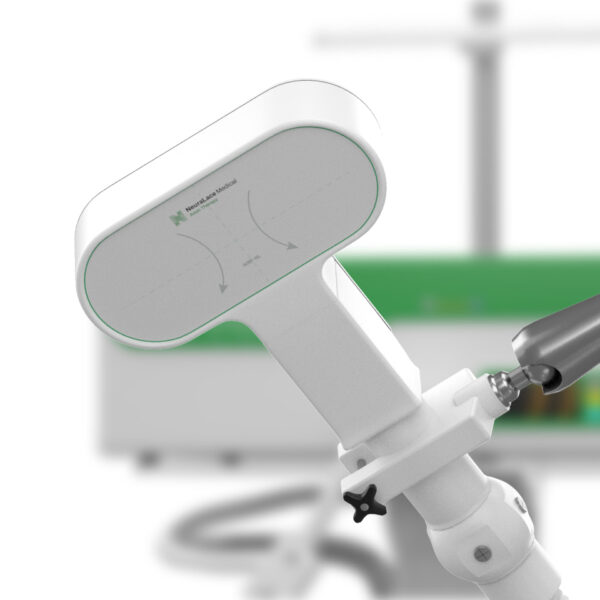
The FDA has approved NeuraLace Medical’s Axon Therapy system that aims to alleviate chronic pain via external nerve stimulation.
Chronic pain is pain lasting for more than three months. An estimated 1.5 billion people worldwide suffer from the condition. The debilitating condition not only increases the burden on public health but also reduces a person’s quality of life. Current treatment options either include addictive opioids, implants, and even LSD. However, NeuraLace Medical’s new device is aiming to change all that. The company recently received 510(k) clearance from the US Food and Drug Administration (FDA) for its new device.
Existing options to treat chronic nerve pain can be ineffective, addictive and invasive. We’re excited to offer patients a method of relief that is non-addictive and non-invasive.
Sean Edwards, Executive Chairman of NeuraLace Medical
The company’s Axon Therapy system aims to treat post-traumatic chronic peripheral nerve pain. This type of chronic pain often results from traumatic injuries such as burns, car accidents, and surgical procedures. Injury damages nerve cells and disrupts signalling between the nerve fibres; thus, resulting in pain.
Through magnetic pulses, the device is able to stimulate A-beta nerve fibres in these patients. After a single session, lasting 15 minutes, patients typically experience a 36% reduction in their pain. Unlike other neuromodulation methods that block the transmission of pain signals, the Axon Therapy system works by re-activating the body’s natural pain management mechanism. العاب لربح المال على النت Although the magnetic pulses are strong enough to stimulate the nerves through a patient’s skin, patients barely feel them. لعبة بلاك جاك
Clinical Trials Underway
The San Diego-based company worked on the device for over 10 years. They tested the device in a few small clinical trials before applying for FDA clearance. كازينو آنلاين Recently, they launched another trial that is expected to enrol 126 patients. Called the AXON-RCT trial, it will compare the effects of Axon Therapy to conventional pain treatment methods.
After 10 years of R&D, we are pleased to have received FDA 510(k) clearance for Axon Therapy and we’re incredibly excited to bring to market a non-opioid treatment option that provides meaningful relief for patients suffering from chronic pain
Shiv Shukla, Founder and CEO of NeuraLace Medical
Moreover, Founder Shiv Shukla aims to bring the device to 22 clinics by next year. The team is also working on a robotic arm that can help hold the device in a precise location for treatment.